It consists of a methylene bridge ch 2 attached to a vinyl group ch ch 2.
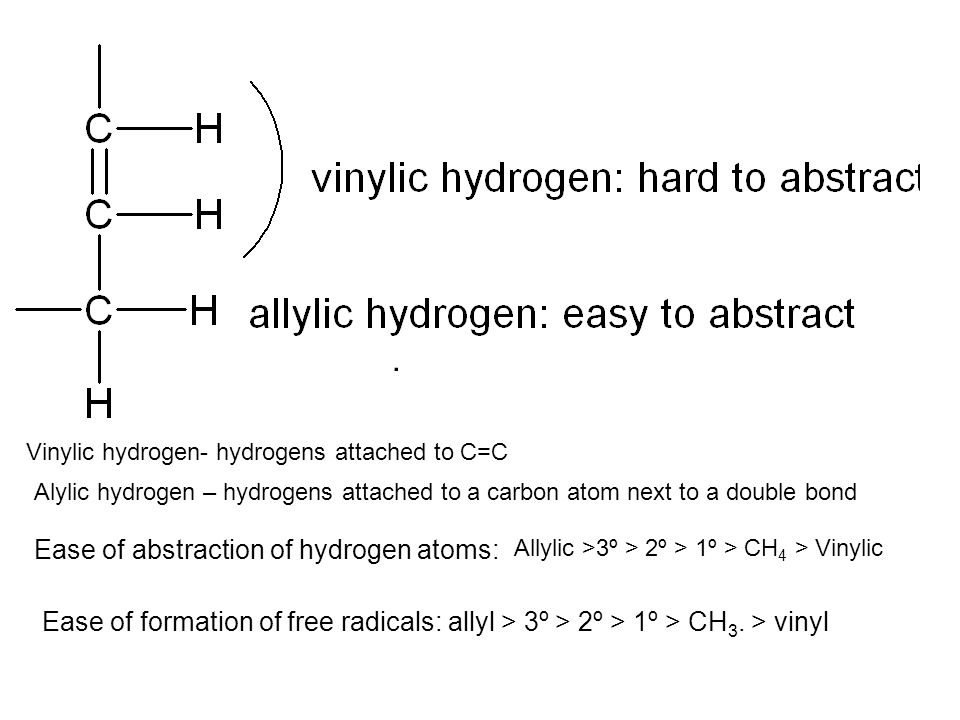
Allylic vs vinylic hydrogen.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
The general formula for allyl is r ch 2 ch ch 2 in which the asterisk carbon atom is an allylic carbon atom.
None of the other hydrogens are vinylic.
The vinylic hydrogens are shown in red.
Allyl groups have three carbon atoms and five hydrogen atoms.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
An allylic hydrogen is a hydrogen atom that is bonded to an allylic carbon in an organic molecule.
The allylic carbon atom is more reactive than normal.
Allyl form a stable carbocation because of the electron delocalization whereas vinylic carbocations are unstable as they lack p character.
The allylic carbon is bonded to a carbon atom which is doubly bonded to another carbon atom.
The allylic position is also like a vinylic position.
It provides plenty of examples including allylic and vinyli.
An allyl group is a substituent with the structural formula h 2 c ch ch 2 r where r is the rest of the molecule.
Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
The name is derived from the latin word for garlic allium sativum in 1844 theodor wertheim isolated an allyl derivative from garlic oil and named it schwefelallyl.
This organic chemistry video tutorial explains how to determine which carbocation is most stable.
Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
A hydrogen atom bonded to an sp 2 carbon of an alkene.